uMed has announced that its pioneering AccessPD Parkinson’s Disease Interactive registry has surpassed 1,000 participants. This milestone marks a significant step forward in advancing research in Parkinson’s Disease by providing life science companies and researchers with access to a large, diverse cohort of patients who are ready to participate in research opportunities.

Enriching Real-World Evidence
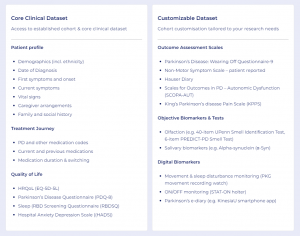
AccessPD offers a unique approach to evidence generation, providing access to data beyond what is routinely available. The interactive registry is designed to enrich real-world evidence with patient experiences, motivations, and outcome assessments that extend beyond traditional clinical efficacy.
Participants contribute data from multiple sources, including their Electronic Health Records (EHRs), genetic tests, wearable devices, and patient-reported outcome surveys. This provides access to a rich source of regulatory-grade data which can then be augmented with the collection of additional custom data as required to answer specific research questions.
Rapid data collection from research-ready participants
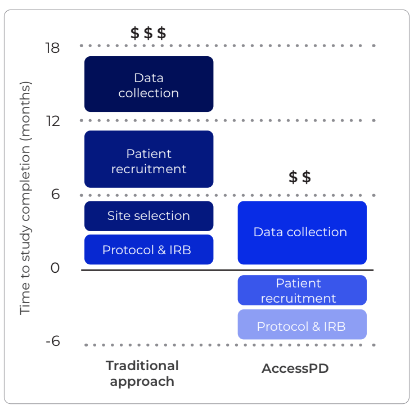
Upon invitation to AccessPD, patients provide their consent to be re-contacted for the collection of additional data or to participate in further research opportunities. This means uMed can rapidly re-contact patients to collect specific data, without having to restart the recruitment and/or consent process from scratch.
In addition, the AccessPD master protocol is ethics-approved so researchers are able to immediately commence observational studies from a pre-consented cohort of patients with a diagnosis of Parkinson’s Disease.
Breaking Down Barriers to Participation
Recruiting and retaining patients for neurodegenerative disease studies, including Parkinson’s Disease, has long been a challenge due to barriers such as travel requirements, communication issues and limited digital access among older populations. AccessPD is uniquely positioned to overcome these hurdles by implementing innovative, patient-centric strategies including:
- Provider-led identification: Patients are identified via their electronic health records which allows for the accurate identification of clinically diagnosed patients. This approach leads to increased engagement and consent rates.
- Multi-Channel engagement: Patients are invited to attend AccessPD on behalf of their healthcare provider via SMS, email and letters, ensuring inclusivity regardless of digital proficiency or technology access.
- Delegated Consent: Accredited nurses are available to guide patients through the consent process via telephone and can provide delegated consent on their behalf, simplifying participation.
- Remote participation: AccessPD provides the opportunity for patients to participate in research from the comfort of their own homes; removing any financial or geographic barriers to taking part.

The success of AccessPD highlights the potential of combining technology with patient-focused engagement methods to address the unique challenges of neurodegenerative disease research.
For more information about AccessPD or to inquire about collaboration opportunities, please contact gabriel.koslover@umed.io