Providing researchers with unparalleled opportunity to access novel endpoints
London, UK, July 10, 2024 – Healthcare evidence-generation and technology company uMed have announced the successful enrollment of over 2,000 participants into their AccessCMD Cardiometabolic Cohort less than 4 months after starting enrollment.
The exponential growth of the cohort within this short time frame highlights the commitment of patients within this disease area to engage in research, and showcases the effectiveness of uMed’s unique model in providing research access that has previously been unavailable.
AccessCMD is a novel decentralised registry and integrated clinical research platform, ethics committee-approved under a master protocol framework. As with all uMed Cohorts, AccessCMD is powered by the Company’s ACCESS Research Platform which is partnered with a vast network of healthcare institutions in the UK and US. This enables rapid and targeted identification of patients via their health record data, and engagement via SMS, email, letter and phone, on behalf of their healthcare institution. Upon invitation to the cohort, patients provide their consent to be re-contacted for the collection of additional data or participate in additional research studies.
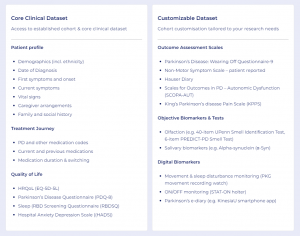
Participants within AccessCMD actively contribute data from their Electronic Medical Records (EMR), genetic tests, wearable devices and patient-reported outcome surveys, offering an unparalleled opportunity for researchers within this disease area to access novel endpoints.
“From the rapid growth we have seen with AccessCMD it is clear that patients are willing and eager to participate in research, they just need to be provided the opportunity to do so, and at uMed we are empowering this unique access.
We achieve this through a combination of tailored communication on behalf of the patients’ trusted healthcare provider, offering research opportunities that are highly relevant to the individual’s health circumstances, and allowing patients to participate from the comfort of their own home” commented Anil Jina, MD. Chief Medical Officer at uMed.
With the rise in global obesity and related diseases, cardiometabolic research is capturing the attention of research groups as they work towards the development of new therapies and tools to address this health crisis.
The potential for these therapies to benefit such a large proportion of the population is evident, however a challenge for researchers at all stages of the therapy lifecycle is the ability to access the required data points from large enough representative sub-cohorts.
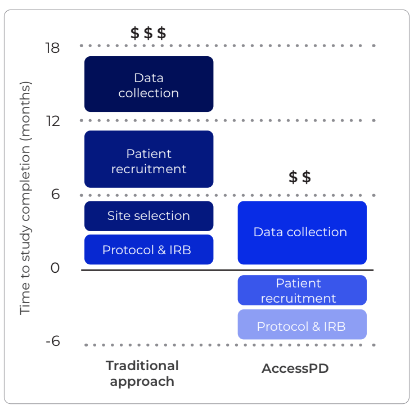
Cardiometabolic disease describes numerous conditions, each interacting through intricate pathways and mechanisms that vary among individuals, many of which remain incompletely understood. Therefore researchers are often faced with the obstacles of finding a sufficient number of patients that meet their very specific study criteria, and obtaining the necessary endpoints needed to answer their research questions. Available databases and registries are static, preventing the easy collection of custom data, and site-based research is time consuming and expensive to set-up.
“We’ve seen the huge impact of recent therapeutic developments within cardiometabolics, and for the many researchers now entering this field, accessibility and speed are key.” commented Dr Matt Wilson, CEO & Founder of uMed. “uMed is bridging the evidence gaps that currently exist between static databases and site-based studies, enabling the rapid collection of custom datasets without the requirement to start studies from scratch. AccessCMD presents researchers with a large cohort of pre-consented patients that researchers can rapidly re-contact to access additional custom endpoints to augment the expanding baseline dataset. ”
“The rate of growth of AccessCMD is compelling and the potential for further growth is exciting. Over a quarter of the population in the UK have risk factors, such high BMI, for serious cardiometabolic diseases. uMed’s integration with healthcare institutions across the UK is providing access to a large number of these patients. If we extrapolate the rate of growth we are seeing in AccessCMD to these wider population numbers, we expect to be able to improve access for patients to relevant research studies and to present researchers with important opportunities to extend their research to groups who have been historically underserved.” commented Dr Mark Toshner, Chief Investigator of AccessCMD
AccessCMD is currently recruiting patients across the UK via uMed’s healthcare network, with plans to launch the Cohort in the US by the end of 2024.