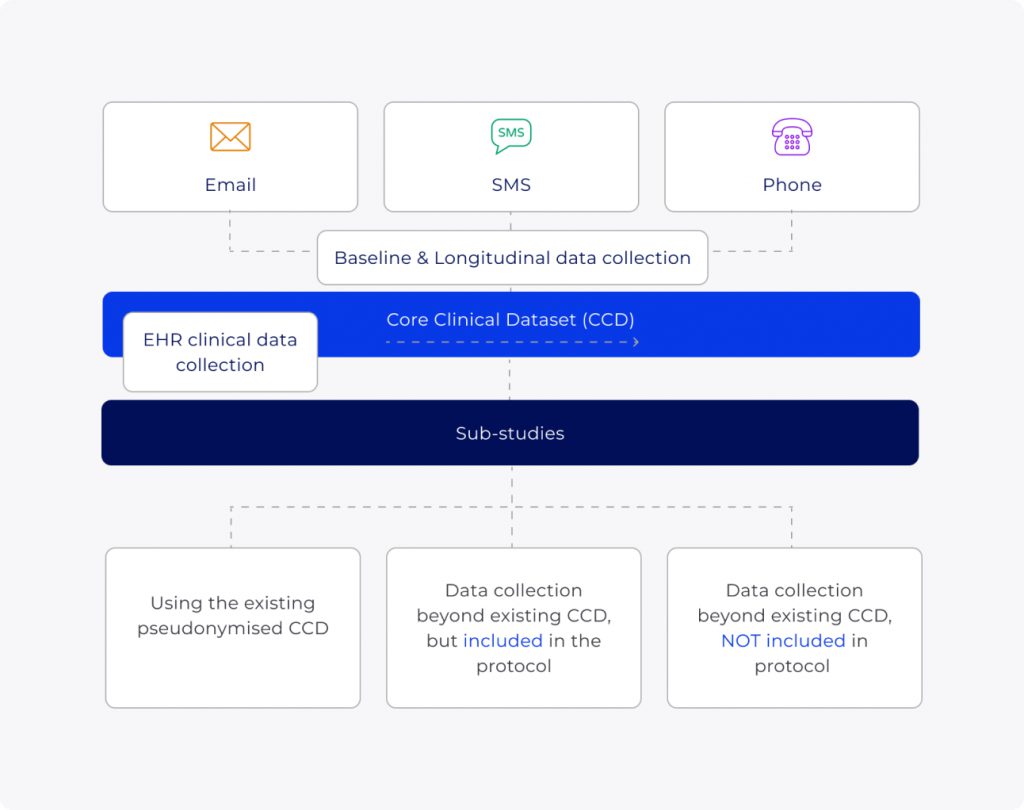
Build a complete picture from comprehensive, regulatory approved data
EMR data
Routinely collected from integration with our network of healthcare providers
Patient generated data
ePROS & home-testing
Clinical data
Objectively measurable parameters, clinician diagnoses, & objective movement data