- uMed launches pioneering AccessCMD patient cohort facilitating access to custom data and insights to help accelerate cardiometabolic disease research
- Cardiometabolic disease is now the leading cause of mortality globally and is placing a huge financial burden on healthcare systems and the economy
- uMed have already recruited over 500 patients into the cohort, achieving over 15% consent rate from the first patient engagements sent, with significant potential for expansion across UK and US healthcare networks
uMed has announced the successful enrollment of the first 500 UK participants into their groundbreaking AccessCMD cohort of patients with cardiometabolic disease.
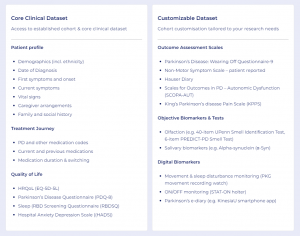
AccessCMD will provide researchers with unique tools to access custom data and insights based on specific research criteria, to accelerate research and understanding across this group of conditions.
Cardiometabolic Disease encompasses a range of conditions including type 2 diabetes, stroke, hypertension, and congestive heart failure. It is now the number one cause of mortality across the globe, accounting for 31% of global deaths. [1]
In the UK the NHS spends around £10 billion a year on type 2 diabetes, approximately 10% of its entire budget,[2] and it is estimated that £7.4 billion is being spent on healthcare related to CVD, with an annual cost to the wider economy of £15.8 billion.[3]
With the prevalence of cardiometabolic diseases on a sharp incline globally, research into this group of diseases is on the rise.
Following the success of initial engagements sent to patients, which resulted in a 15% consent rate, uMed is now rolling out further engagements via their extensive UK healthcare provider network.
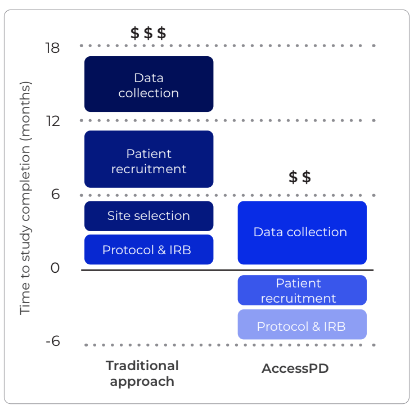
uMed’s unique approach to patient identification, outreach, and consent, combined with fully decentralised data collection, mitigates biases that often arise in research due to factors such as patient location, socioeconomic circumstances, race, gender, and age.
‘The conversion rate we achieved from our first engagements is compelling; if we extrapolate this rate to the eligible patient population covered by our healthcare provider network in the UK, we’ll be able to offer thousands of patients the opportunity to participate in research and very quickly build a unique and rich source of information in Cardiometabolic Disease” commented Anil S. Jina, MD, Chief Medical Officer & President North America, at uMed.
Unlike traditional registries that are static and do not easily allow for the generation of custom data, AccessCMD enables re-engagement with patients that have already consented to participate in additional studies. This allows for the rapid collection of custom data that holds the answer to specific research questions, in the form of electronic health records, clinical outcomes, patient-reported outcomes and biosamples.
Dr Mark Toshner, Consultant Respiratory Physician at the University of Cambridge, and AccesssPD Principal Investigator, commented “The potential that AccessCMD holds for research is extensive. The prevalence of these diseases has grown at a substantial rate over the past 20 years and the consequence is a huge burden on the populations’ health and our healthcare systems, so there is much research to be done to understand the mechanisms contributing to these poor health outcomes.
“Conducting research at a large-scale with patient groups that represent the entire population is essential for developing a true understanding of the disease and underlying burden or unmet needs. The challenge for researchers is finding enough patients that meet the very specific criteria to conduct robust studies.’
“AccessCMD provides researchers with access to custom data to answer their key research questions, as and when required, and even enables the running of standalone studies within the infrastructure of the existing cohort. This will significantly expedite the development of understandings and treatments to reduce the burden of these diseases.”
The launch of AccessCMD follows the success of uMed’s inaugural AccessPD cohort study, which has so far recruited over 500 patients diagnosed with Parkinson’s Disease in the UK,
Additionally, the company has recently begun the roll-out of engagements to patients with Interstitial Lung Disease, with nearly 100 patients in the UK now consented into their AccessILD cohort.
The swift succession of AccessCMD underscores the popularity of uMed’s Cohort Platform Studies amongst both patients and healthcare providers, highlighting the value for researchers within cardiometabolic disease and many more disease areas.
Plans are underway to launch AccessPD, AccessILD and AccessCMD in the USA in 2024.
[1] https://www.roche.com/solutions/focus-areas/cardiometabolic
[2] https://www.england.nhs.uk/2022/03/nhs-prevention-programme-cuts-chances-of-type-2-diabetes-for-thousands/
[3] https://www.gov.uk/government/publications/health-matters-preventing-cardiovascular-disease/health-matters-preventing-cardiovascular-disease#:~:text=Yearly%20healthcare%20costs%20in%20England,economy%20of%20%C2%A315.8%20billion.