Patient Perspectives

"It's been 5 years since my diagnosis but this is my first time taking part in Parkinson's research because of my poor mobility. Being able to take part despite having no internet is a big positive and your nurses make this possible."
Carole Henrickson, 75, Hull
AccessPD participant

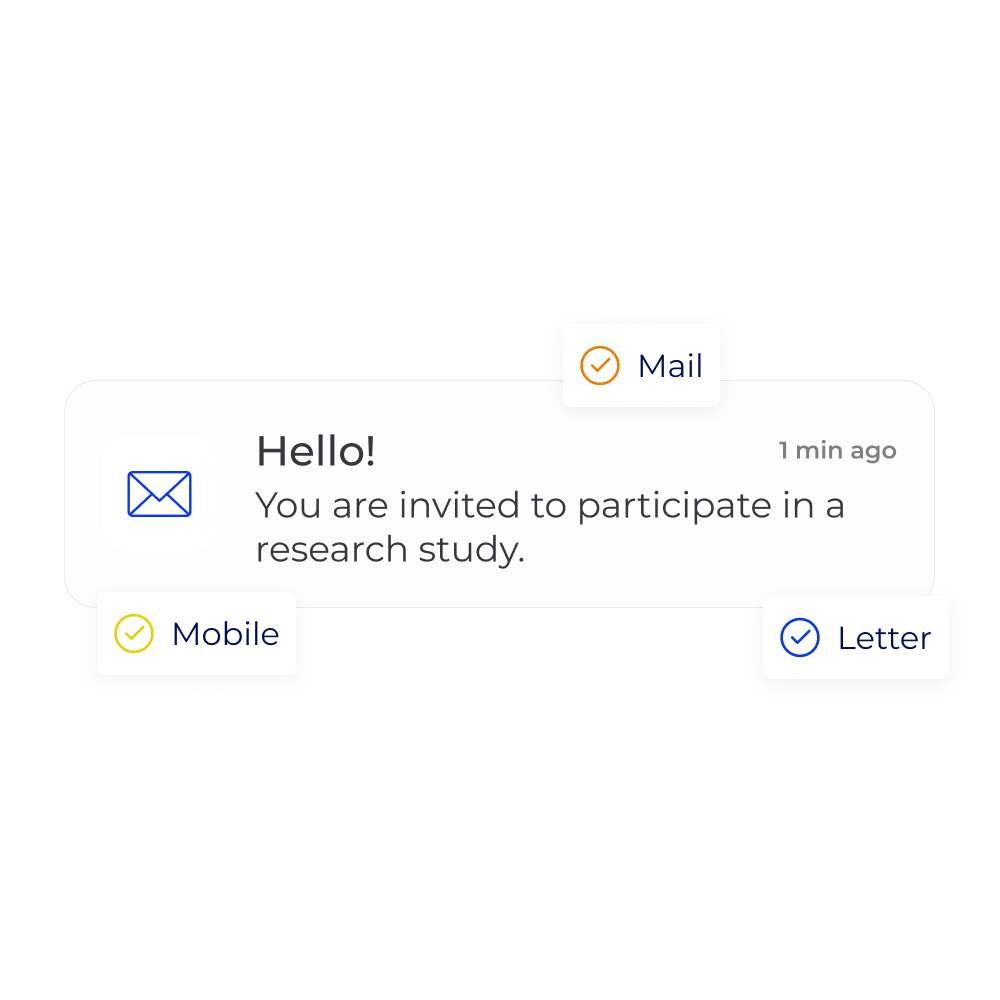
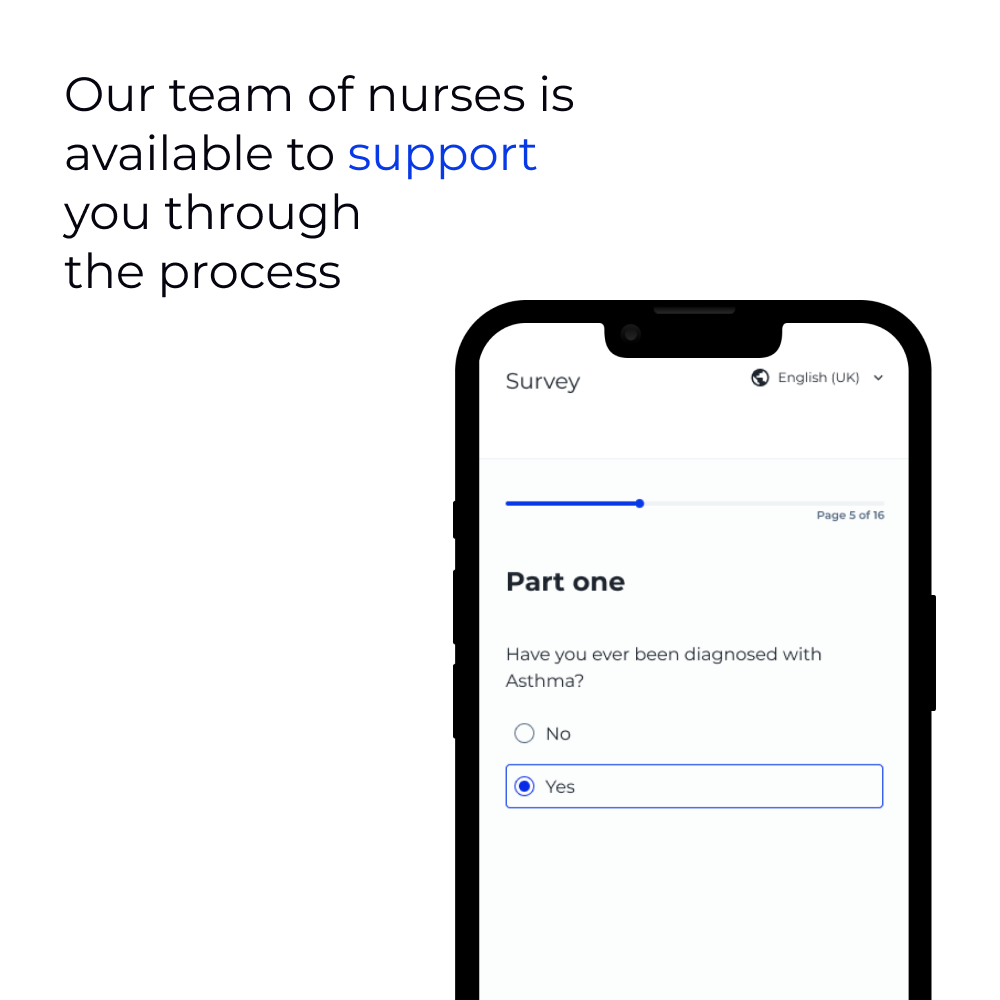
"The whole process went smoothly, uMed handled everything so I was able to participate entirely from home and didn’t have to travel. The team were extremely helpful, friendly and professional, and were available to answer any questions I had about the study. This made me, and my wife, feel completely at ease with taking part."
Kevin Quinn, Liverpool
Study participant