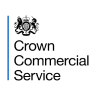The agreement details how uMed will process data on behalf of the practice, both for research and direct care programmes. This includes processing to:
a) Match potential subjects in the practice population with study opportunities for review by the practice.
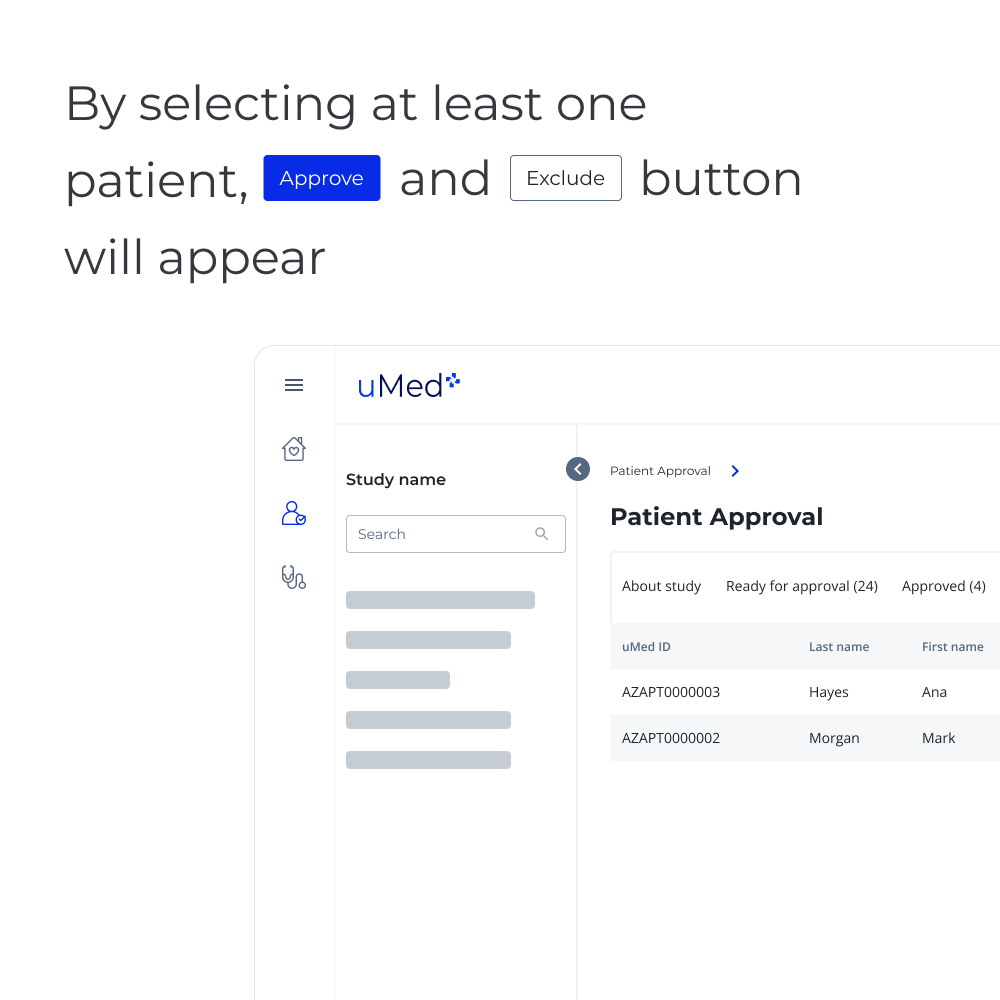
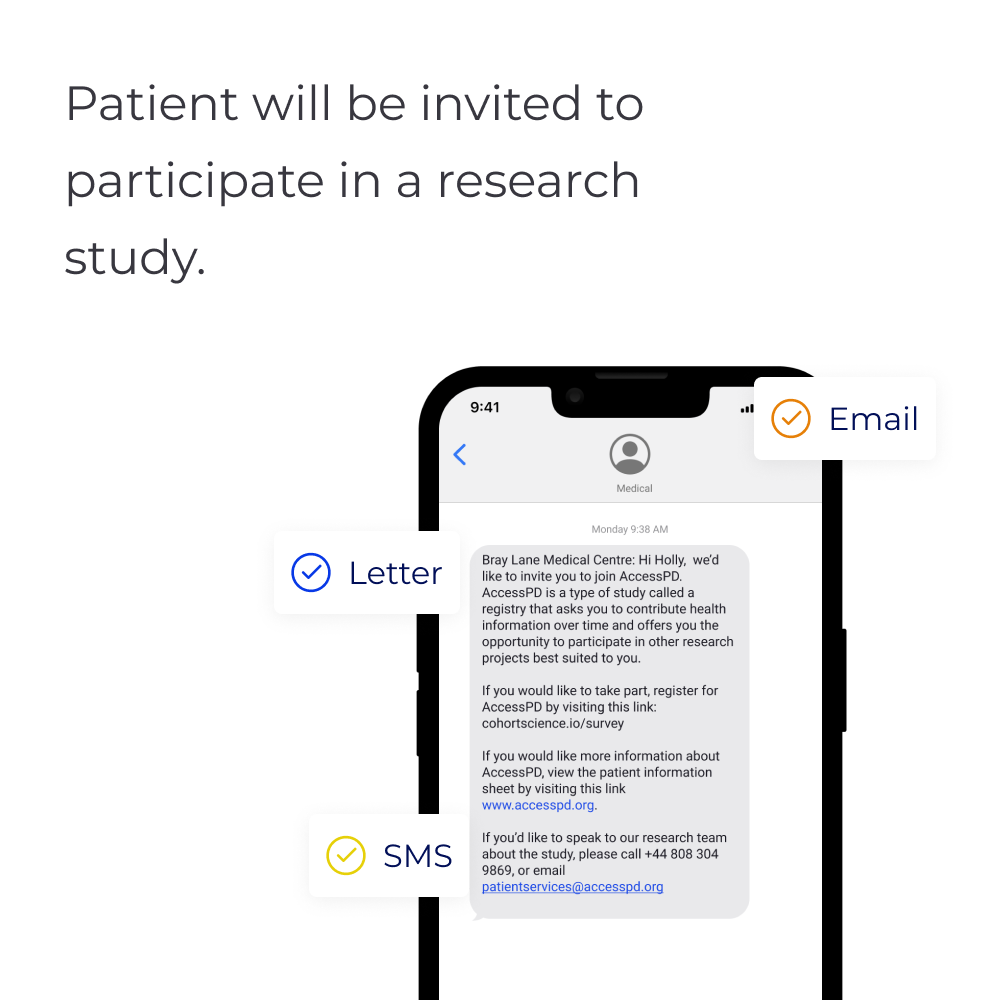
b) If approved by the practice, engagement of those patients on behalf of the practice to support recruitment and data capture.
c) If approved by the practice, linkage of outcomes from the clinical record to the study case report form (CRF).
It is important to note that this is not a data-sharing agreement. As a data processor, uMed cannot share or utilise practice data unless explicit permission is obtained from the practice (the data controller). In the same way, other NHS processors such EMIS, Apollo, Accurx, and other technology vendors cannot use practice data outside of that defined in their service agreement with practices.
The DPA is required to legally allow your practice to share patient data in order to provide patients with individual care and offer them research opportunities to take part in. This is also the legal basis for uMed to process patient data on behalf of their Healthcare Providers (the controller of the data).