London, UK – Thursday 1 May — uMed, a leading innovator in healthcare technology, is proud to announce the launch of SnapACCESS — a revolutionary new platform designed to transform how pharmaceutical companies access and leverage patient data to support faster, smarter drug development. The first rollout will focus on the critical cardiometabolic disease (CMD) area, particularly diabetes and obesity.
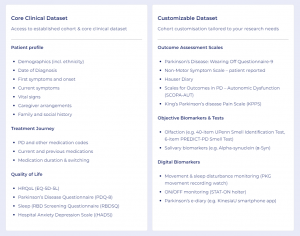
SnapACCESS empowers pharmaceutical teams with compliant, agile access to highly targeted, clinically confirmed patient groups, including detailed insights into patients’ real-world treatment journeys — such as the medications they are currently prescribed. This uniquely enables organizations to optimize study design, site selection, and early feasibility assessments with unprecedented speed and precision.
“SnapACCESS solves a problem common across academia and industry,” said Professor Mark Toshner, Director at Cambridge Heart & Lung Institute. “By combining rapid, compliant access to real-world clinical data with direct patient engagement, we are not just speeding up research — we are elevating its quality. This is a transformative step towards more patient-centered, data-driven drug development.”
A New Standard for Integrated Data and Patient Engagement
Unlike traditional retrospective datasets or self-reported patient panels, SnapACCESS offers a dynamic, twofold advantage:
- Full access to linked electronic health record (EHR) data, delivering real-time insights into patients’ medical histories, comorbidities, treatment pathways, and outcomes.
- Direct patient engagement through uMed’s dedicated nurse support and patient services team, ensuring patients fully understand research questions and allowing for adaptive follow-up interactions to capture deeper insights.
This approach not only ensures regulatory-grade compliance (IRB/REC approved, GDPR/HIPAA compliant) but also enables repeatable, high-quality interactions with pre-consented patients — a vital advantage for iterative research and longitudinal studies.
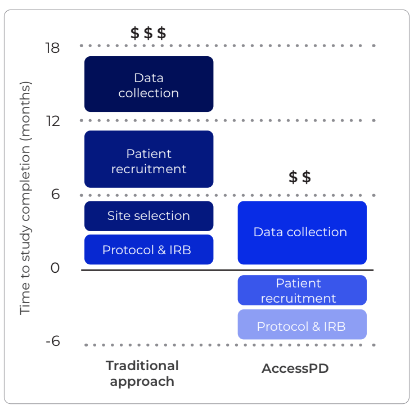
Delivering Actionable Insights in Under 4 Weeks
SnapACCESS is built for agility:
- Insights delivered in under four weeks
- Interactive, AI-powered dashboards for real-time data exploration
- Flexible question structures and the ability to re-engage patient cohorts
- Rapid segmentation by clinical markers, treatments, and outcomes
Pharmaceutical companies can quickly refine their strategies, validate market insights, enhance clinical narratives, and accelerate trial readiness — driving more effective therapies to market faster.
Focused Launch with Expansion Plans
SnapACCESS initially targets diabetes and obesity patient cohorts, supported by uMed’s growing network of over 5,500 active patients, with ongoing enrollment expanding by 1,000+ patients per month.
Future expansions will broaden access to additional therapeutic areas across the cardiometabolic spectrum and beyond.
About uMed
uMed is a technology-driven healthcare platform committed to transforming the future of clinical research by enabling healthcare providers and life science organizations to drive faster, smarter, patient-centered studies without burdening clinical teams.
Contact:
Dr. Matt Wilson, MD
matt.wilson@umed.io
www.umed.io